On 16 November, 2020, China’s Ministry of Ecology and Environment published a revision of the Guidance on New Chemical Substance Environmental Management Registration (Announcement No. 51 of 2020) and relevant forms with instructions on how to fill them in. This is in line with the amendment of the Measures on New Chemical Substance Environmental Management Registration (Decree No. 12 of the Ministry of Ecology and Environment; published in April 2020). The revised guidance takes effect on 1 January, 2021. Here are the key points of the revision:
Registration application forms
- Quantitative assessments are required in the risk assessment reporting. More specific requirements, including the assessments of hazards and exposure effects of chemicals, are defined for the reporting as well.
- Detailed requirements of socio-economic benefit analysis reporting of highly hazardous chemical substances are introduced (e.g. noticeable benefits compared to other chemicals used for the same purposes).
- The information protection period for information other than identification information, such as chemical names, lasts until the entity that has registered the chemical cancels the registration or the government publishes the information for other important reasons (the protection period for identification information is five years from the first registration or notification date).
- Specific requirements for environmental management registration of chemical substances for new purposes are introduced, including registering the chemicals in the regular registration procedure.
Data submission requirements
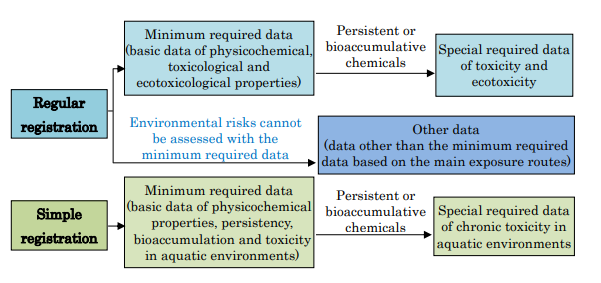
- Minimum required data specifies data normally required for the regular and simple registration. When environmental risks cannot be assessed with this data, additional data is required to be submitted (see the diagram below).
- Detailed requirements of data submission based on data classification are defined. When risks can be controlled, costly tests are not required so that companies will bear less cost.
- Special required data, which is defined in the minimum required data, should be based mainly on test reports; test reports should be used preferentially over other data as the data source. When relevant tests cannot be performed, other data can be used.
Relevant forms
- Fourteen forms are attached to the revised guidance, including the Registration Application Form and Initial Operation Status Report Form. Specific reporting requirements for post-registration management are defined.
You can see the full text of the revised guidance and the relevant forms with instructions at
http://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202011/t20201119_808843.html.
 China releases revised guidance for new chemical registration
China releases revised guidance for new chemical registration 
























